Lithium Battery Manufacturing
You see lithium batteries everywhere—from smartphones to electric vehicles. Lithium Battery Manufacturing involves several precise steps to ensure high performance and safety. Since Akira Yoshino designed the first lithium battery prototype in 1985, and Sony commercialized it in 1991, the technology has evolved rapidly. Today’s batteries offer high energy efficiency, quick charging, and long operational life. In 2023, global production reached 2.5 TWh, supporting 14 million EV sales and over 90% market share.
| Year | Global Battery Production (TWh) | EV Sales (millions) |
|---|---|---|
| 2022 | 1.72 | 10.4 |
| 2023 | 2.5 | 14 |
Key Takeaways
- Lithium batteries power many devices, from smartphones to electric vehicles, and their manufacturing involves precise steps to ensure safety and performance.
- Understanding battery materials, like cathodes and anodes, is crucial. Different materials affect energy density, cycle life, and overall battery efficiency.
- Quality control is vital in battery production. Regular testing and strict protocols help ensure safety and reliability throughout the manufacturing process.
- Automation and cleanroom environments enhance production efficiency and reduce contamination risks, leading to higher quality batteries.
- Sustainability is a growing focus in battery manufacturing. Supporting recycling and reducing reliance on rare materials can help create a cleaner future.
Lithium Battery Materials

Lithium batteries rely on several key materials. Each part plays a unique role in how the battery stores and delivers energy. You can explore these materials to understand how they affect battery performance and safety.
Cathode
The cathode acts as the source of lithium ions. You find different cathode materials in batteries, each with specific advantages. The lithium nickel manganese cobalt (NMC) cathode holds the largest market share. NMC offers high energy density and long cycle life, which makes it popular for electric vehicles and advanced energy storage. Lithium iron phosphate (LFP) dominates in Asia Pacific, especially for mass-market EVs and grid-scale energy storage systems. Other cathode types include NCA, LCO, and LMO, each serving niche applications.
| Cathode Material | Market Share | Growth Rate (CAGR) | Key Applications |
|---|---|---|---|
| Lithium Iron Phosphate (LFP) | Dominant in Asia Pacific | >23% | Mass market EVs, Grid Scale ESS |
| Lithium Nickel Manganese Cobalt (NMC) | Largest market share | High CAGR | Electric vehicles, advanced energy storage |
| Lithium Nickel Cobalt Aluminum Oxide (NCA) | Niche market | High CAGR | Premium EVs (e.g., Tesla) |
| Lithium Cobalt Oxide (LCO) | Niche market | N/A | High-end consumer electronics |
| Lithium Manganese Oxide (LMO) | Supporting role | N/A | High power, low capacity applications |
The global cathode materials market reached $38.47 billion in 2024. China leads with a 55.11% share, and the market is projected to grow to $135.73 billion by 2032.
Anode
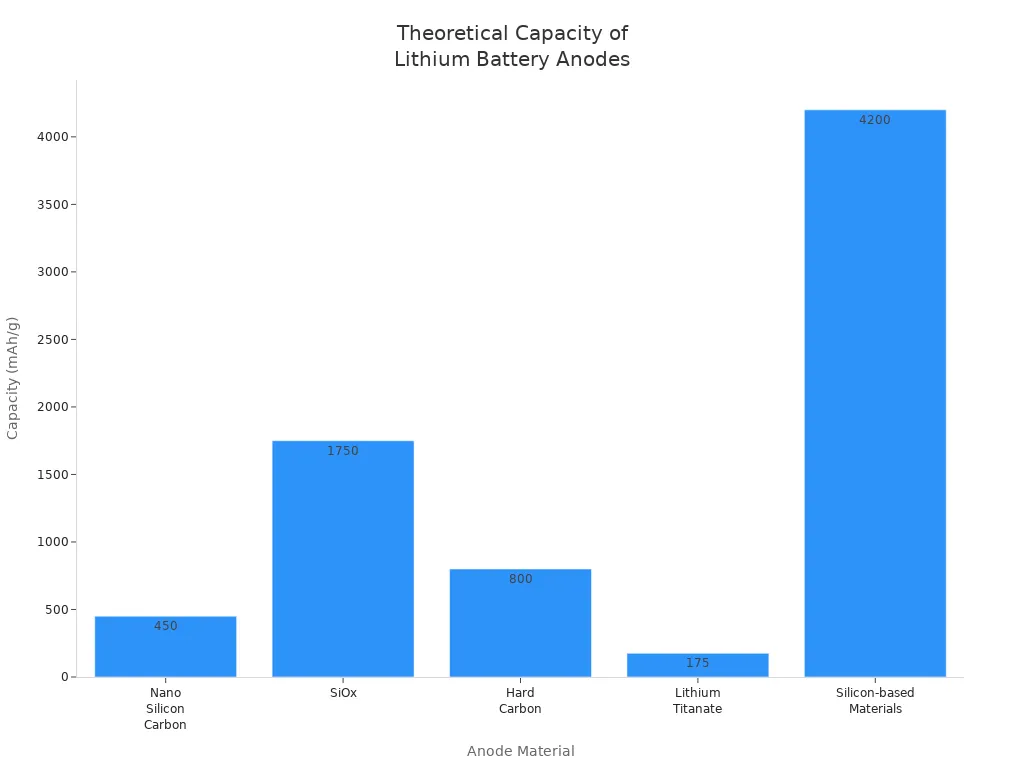
The anode stores lithium ions during charging. You see graphite as the most common anode, but new materials like silicon-based compounds are emerging. These materials offer higher theoretical capacity, which means batteries can store more energy. Nano silicon carbon and SiOx show promise, but researchers continue to improve their cycle life and efficiency.
| Anode Material | Theoretical Capacity (mAh/g) | Initial Efficiency | Cycle Life Characteristics |
|---|---|---|---|
| Nano Silicon Carbon | 400-500 | >80% after 600 cycles | Limited specific energy improvement |
| SiOx | 1500-2000 | Lower than graphite | High volume expansion, ongoing research for initial capacity reduction |
| Hard Carbon | 800 | Good rate performance | >60% retention after 2000 cycles at 10°C |
| Lithium Titanate | 175 | High initial efficiency | Excellent cycle life, low specific energy |
| Silicon-based Materials | 4200 | Safer lithium intercalation | Research ongoing for practical applications |
Electrolyte
Electrolytes allow lithium ions to move between the anode and cathode. You benefit from electrolytes with high conductivity and low internal resistance, which help maintain operating voltage and current. The composition of the electrolyte affects how well the battery performs during fast charging and discharging. Stability and temperature range also impact the aging process and overall cycle life.
Separator
The separator keeps the anode and cathode apart, preventing short circuits. You rely on separators with strong physical strength and high porosity for efficient ion transport. Advanced separators use ceramic coatings and high melting point polymers to improve safety. If the separator melts at high temperatures, it shuts down ion flow and helps prevent thermal runaway.
| Property | Description |
|---|---|
| Shutdown Mechanism | Separators melt at high temperatures, closing pores to stop ion transport and current flow. |
| Breakdown Mechanism | Loss of separator integrity allows direct contact between electrodes, leading to thermal runaway. |
| Desired Temperature Range | A wide gap between shutdown and breakdown temperatures is crucial to delay thermal runaway. |
| Physical Strength | Separators must maintain mechanical and electrical separation under high temperature conditions. |
| Electrolyte Uptake | Higher uptake lowers cell resistance, enhancing efficiency. |
| Porosity | A highly porous structure is essential for effective ion transport. |
| Material Improvements | Ceramic-coated and high melting point polymers improve thermal stability and abuse tolerance. |
Other Parts
You find several other essential components in lithium batteries. Current collectors support the electrodes and ensure electrons flow to the external circuit. The casing protects the battery and provides structural integrity. Binders hold the materials together, maintaining the battery’s shape and function.
| Component | Function |
|---|---|
| Anode | Stores lithium ions when the battery is charged and serves as the starting point for energy flow. |
| Cathode | Source of lithium ions for chemical reactions, determining capacity and voltage of the battery. |
| Electrolyte | Medium for the flow of lithium ions between anode and cathode, enabling charging and discharging. |
| Separator | Prevents anode and cathode from touching, avoiding short circuits and potential hazards. |
| Current Collectors | Support electrode materials and ensure efficient electron flow to the external circuit. |
| Casing | Protects internal components and provides structural integrity to the battery. |
| Binder | Holds battery components together, ensuring structural integrity. |
Lithium Battery Manufacturing
You play a key role in understanding how lithium battery manufacturing works. Each stage in the process shapes the battery’s performance, safety, and reliability. Let’s explore the steps you encounter in modern battery production.
Material Mixing
You start with material mixing, which sets the foundation for battery quality. The process involves several critical steps:
- Add the active substance and conductive agent, mixing slowly to ensure even distribution.
- Introduce the glue, which acts as a binder for the mixture.
- Control the revolution and rotation speed of the mixing equipment. The linear speed of dispersion must stay above 17 meters per second.
- Test the particle size and viscosity of the slurry regularly. These properties depend on solid content, material type, and the order in which you add ingredients.
- Transfer the mixed slurry to the next stage, making sure to sieve it and remove large particles or ferromagnetic substances.
- For the negative electrode, use an aqueous mixing process with deionized water. You must keep conductivity at or below 1 microsiemens per centimeter.
Tip: Careful control during material mixing helps prevent defects and ensures consistent battery performance.
Slurry Preparation
You move to slurry preparation, where precision matters. Industry standards guide you through each step:
- Pretreat the active material, conductive agent, and binder by drying and sieving.
- Blend solid components and solvent in exact proportions.
- Use high-speed dispersion equipment, such as planetary mixers, to achieve a uniform mixture.
- Remove air bubbles through degassing treatment, which improves coating quality.
- Filter the slurry with fine screens to eliminate large particles.
You must monitor key parameters:
- Solid content ranges from 50% to 70% for positive electrode slurries and 40% to 60% for negative ones.
- Viscosity should stay between 4000 and 8000 mPa·s for positive slurries, and 1000 to 5000 mPa·s for negative slurries.
- Fineness, measured by D50 value, should fall between 1 and 10 micrometers.
Factors like stirring speed, temperature, and the order of adding materials affect the slurry’s final properties. You need to adjust these variables to achieve the best results.
Coating & Drying
You apply the prepared slurry onto metal foils using advanced coating machines. The drying process removes solvents and solidifies the electrode layer. The quality of coating and drying directly impacts battery performance. You can see how different parameters affect the electrode:
| Parameter | Effect on Electrode Properties |
|---|---|
| Calender Gap | Wider gaps create thicker, denser electrodes with less porosity. |
| Roller Speed | Faster speeds increase mass loading and reduce porosity, improving ionic resistance. |
| Energy Efficiency | Semidry methods cut solvent use by over half, saving energy and space in production. |
Note: Uniform coating and controlled drying prevent defects and improve battery consistency.
Calendering & Slitting
You enhance the electrode’s density and function through calendering. This step presses the dried film to achieve the desired thickness and porosity. You then slice the calendered foil into precise sizes for battery design. Best practices include:
| Process | Description | Best Practices |
|---|---|---|
| Calendering | Press the dried film to boost density and battery function. | Control thickness and porosity for optimal ion movement. |
| Electrode Slicing | Cut calendered foil to fit battery specifications. | Ensure accurate cuts and smooth edges to prevent short circuits. |
You must balance density and porosity to allow ions to move easily, which improves battery efficiency.
Cell Assembly
You assemble the battery cell using one of several techniques. Each method affects the battery’s shape and performance:
| Assembly Technique | Description |
|---|---|
| Stacking Process | Cut anode, cathode, and separator sheets individually, then stack them to form the cell. |
| Z-folding Process | Cut anodes and cathodes into sheets, keep the separator continuous, and use Z-folding to separate layers. |
| Rolling Process | Roll four sheets (anode, separator, cathode, separator) together onto a base to create the cell shape. |
You must align and seal each component precisely to avoid defects and ensure safety.
Electrolyte Filling
You fill the assembled cell with electrolyte, which enables ion movement between electrodes. Accurate filling and sealing are essential. You test for leaks and confirm that the electrolyte reaches all parts of the cell. This step supports efficient charging and discharging.
Formation & Aging
You begin the formation process by charging and discharging the battery for the first time. Aging follows, allowing the battery to stabilize. These steps provide several benefits:
- Stabilize the solid electrolyte interface (SEI) film, reducing side reactions and improving cycle life.
- Release internal stress, preventing mechanical failures.
- Allow gases to escape, preventing bulging and improving interface contact.
- Screen out defective cells, ensuring only reliable batteries move forward.
- Balance electrolyte distribution, which boosts ion transmission efficiency.
Callout: Formation and aging are critical for long-term reliability in lithium battery manufacturing.
Quality Control
You implement strict quality control measures throughout lithium battery manufacturing. These steps protect product safety and performance:
- Maintain clean facilities to prevent contamination.
- Use automation to reduce human error and standardize production.
- Test samples regularly to ensure quality.
- Inspect raw materials for purity and quality.
- Monitor electrode production for thickness and adhesion.
- Check cell assembly for alignment and sealing integrity.
- Verify electrolyte filling and sealing with leak detection tests.
- Track voltage, capacity, and resistance during formation and aging.
You also perform:
- Electrical testing to measure charge storage and delivery.
- Safety testing to evaluate response to overheating and short circuits.
- Environmental testing to assess stability under extreme conditions.
Tip: Rigorous quality assurance at every stage ensures you deliver safe, high-performance batteries.
You see that lithium battery manufacturing demands engineering rigor and attention to detail. Each step, from material mixing to quality control, shapes the final product’s reliability and efficiency. By mastering these processes, you help drive innovation and support the growth of battery-powered technology.
Lithium Battery Production Process

Automation
You see automation as a driving force in the lithium battery production process. Automated systems help you achieve consistent quality and high efficiency. Modern factories use advanced technologies to connect, assemble, and test battery cells. For example, the BLS 500 Laser Welding System creates precise connections between cells, reducing errors. Graphical programming tools let you adapt machines for different battery designs. Virtual commissioning tools allow you to test control programs before assembly, saving time and resources. OPC UA communication ensures smooth data exchange between machines, which boosts overall performance.
| Technology | Contribution to Efficiency and Consistency |
|---|---|
| BLS 500 Laser Welding System | Precise cell connections for better accuracy |
| Graphical Programming Tool | Easy adaptation for various battery applications |
| Virtual Commissioning Tool | Faster setup by testing programs before assembly |
| OPC UA Communication | Continuous data exchange for improved system performance |
Automation helps you reduce human error and maintain high standards in every batch.
Cleanroom
You work in cleanrooms to prevent contamination during battery production. Cleanrooms use strict standards to control dust, humidity, and temperature. Electrode coating and material handling require ISO Class 6 environments. Cell manufacturing takes place in ISO Class 7 cleanrooms. Packaging and quality control use ISO Class 8 standards. Air change rates range from 60 to 240 times per hour, keeping the air clean. Humidity stays below 1%, and temperature remains stable within ±1°C. ESD protection includes conductive flooring and grounding equipment. Physical separation and unidirectional workflows prevent cross-contamination. You must follow ISO/TS 16949 and other regulations to ensure safety and quality.
- ISO Class 6: Electrode coating and active material handling
- ISO Class 7: Cell manufacturing operations
- ISO Class 8: Packaging and quality control
- Air change rates: 60–240 per hour
- Humidity: Below 1%
- Temperature: ±1°C stability
- ESD protection: Conductive flooring and grounding
- Cross-contamination prevention: Physical separation and workflow design
- Regulatory compliance: ISO/TS 16949
Cleanrooms protect your batteries from dust and moisture, which improves reliability.
Safety
You follow strict safety protocols to prevent accidents and contamination. Proper storage uses approved containers and avoids extreme temperatures, which reduces thermal runaway risks. Charging protocols require manufacturer-approved chargers and constant monitoring of voltage and temperature. Emergency response plans define evacuation procedures and fire suppression methods. You receive regular training on handling, storage, and charging to stay aware of risks.
| Safety Protocols | Description |
|---|---|
| Proper Storage | Approved containers, avoid extreme temperatures |
| Strict Charging Protocols | Use correct chargers, monitor voltage and temperature |
| Emergency Response Plans | Evacuation procedures, fire suppression methods |
| Employee Training | Regular courses on handling, storage, and charging |
Safety measures protect you, your coworkers, and the environment during every step of production.
Battery Production Challenges
Material Sourcing
You face several challenges when sourcing materials for battery production. The supply chain depends on a few countries for critical minerals, which creates risks if political situations change. Mining activities can harm local ecosystems and cause pollution. The industry grows quickly, but you may struggle to find enough skilled workers. High costs and limited technology also make it hard to scale up production.
- Supply chain constraints can disrupt access to lithium, cobalt, and nickel.
- Environmental impact from mining affects air, water, and soil quality.
- Workforce gaps slow down battery production and innovation.
- High costs and technology limits restrict large-scale deployment.
You need to address these sourcing issues to maintain reliable battery production and improve overall performance.
Cost
You see cost as a major challenge in battery production. Prices for lithium and other raw materials change often. These fluctuations make it hard for manufacturers to keep profit margins stable. Electric vehicle makers struggle to offer affordable products when material costs rise. Companies like Tesla and Panasonic work hard to secure supplies and reduce the effects of price swings.
- Fluctuating lithium prices impact profit margins.
- Volatile costs make it difficult to keep electric vehicles affordable.
- Manufacturers must secure supplies to protect against price changes.
Environment
Battery production affects the environment in many ways. You need to consider carbon emissions, water usage, pollution, and recycling challenges.
| Environmental Impact | Description |
|---|---|
| Carbon Emissions | Production creates high emissions, with 46% of EV emissions from manufacturing. |
| Water Usage | Making one tonne of lithium uses about 2 million tonnes of water, causing depletion in regions like South America. |
| Pollution | Extracting metals like nickel and cobalt contaminates land and water. |
| Recycling Challenges | Only 5% of batteries get recycled due to high costs and inefficiencies. |
You can help by supporting sustainable mining, using renewable energy, and improving recycling technologies. Decarbonizing the supply chain with low-carbon hydrogen and biofuels also reduces environmental harm.
Safety
You must follow strict safety measures during battery production. Buying from reputable suppliers ensures high safety standards. You need to use the correct battery sizes and voltage outputs for each device. Proper storage and handling prevent damage and exposure to extreme conditions. Battery management systems monitor charging and discharging to avoid hazards.
- Purchase from trusted manufacturers.
- Use correct battery sizes and voltage outputs.
- Store and handle batteries carefully.
- Implement battery management systems for safe charging and discharging.
To protect battery cells, you rely on built-in safety mechanisms. These systems detect problems like overcharging or high temperatures and trigger protective actions to prevent accidents.
Trends & Innovations
Solid-State
You see solid-state batteries leading the next wave of lithium battery innovation. These batteries use solid electrolytes instead of liquid ones. This change brings major improvements in safety and energy density. You can compare solid-state batteries with conventional lithium-ion batteries in the table below:
| Feature | Solid-State Batteries | Conventional Lithium-Ion Batteries |
|---|---|---|
| Energy Density | Up to 500 Wh/kg | 250-300 Wh/kg |
| Safety | Non-flammable solid electrolytes | Flammable liquid electrolytes |
| Lifespan | 8,000 to 10,000 charge cycles | 1,500 to 2,000 charge cycles |
| Charging Speed | Higher currents without degradation | Slower charging rates |
| Thermal Management | No need for complex systems | Requires thermal management systems |
| Performance in Extremes | Stable from -30°C to 100°C | Performance can degrade in extreme temps |
| Manufacturing Complexity | Higher costs and complexity | More established manufacturing processes |
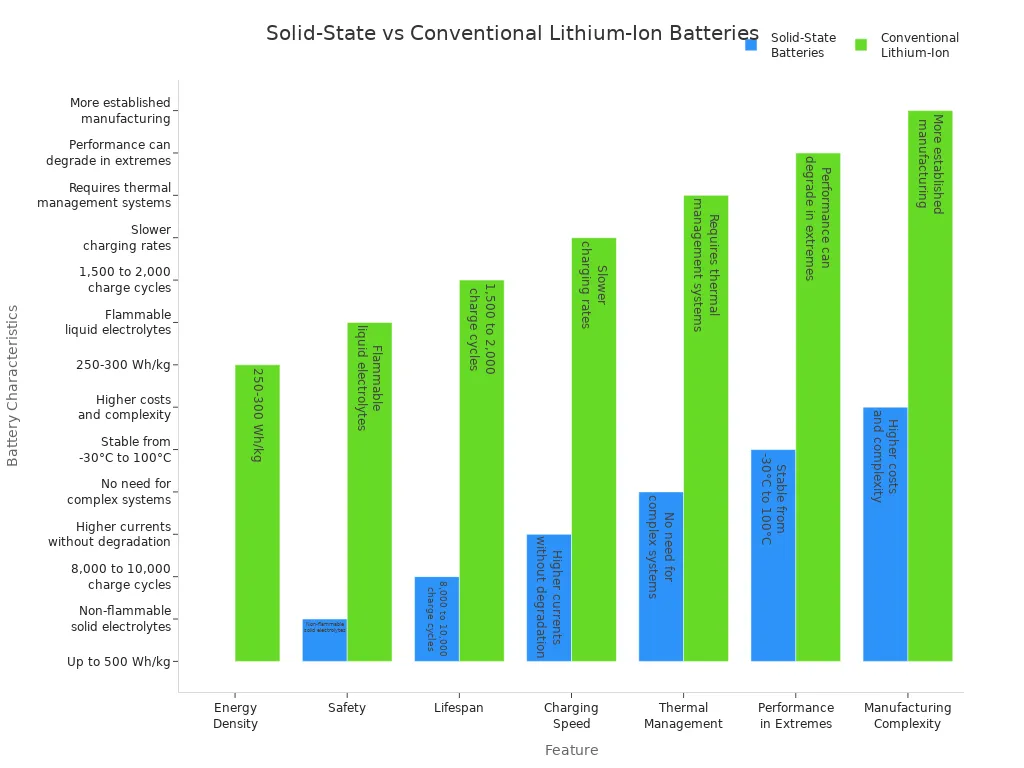
You benefit from solid-state batteries because they reduce fire risks and lower chances of thermal runaway. Higher energy density means longer driving ranges for electric vehicles. You also see better performance in extreme temperatures.
Recycling
You play a key role in shaping battery recycling trends. Modern recycling methods help recover valuable materials and reduce waste. You can review the main recycling techniques in the table below:
| Method | Advantages | Disadvantages |
|---|---|---|
| Pyrometallurgy | Can process diverse battery types effectively | Energy-intensive, generates emissions, struggles with lithium recovery |
| Hydrometallurgy | Less energy-intensive, effective for certain metals | Produces liquid waste requiring treatment |
| Direct Recycling | Streamlines process, eliminates intermediate steps | May require customization based on cathode type |
You see new technologies like BRAWS, which use only water and carbon dioxide. This method avoids chemicals and high heat, allowing for more lithium recovery than older processes.
Sustainability
You notice sustainability shaping every part of lithium battery manufacturing. Companies focus on efficient recycling and building infrastructure for a circular economy. You see research aiming to reduce reliance on rare materials and improve safety. Efforts to repurpose batteries and develop cost-effective recycling methods continue to grow. The global lithium market is projected to reach $74.81 billion by 2030, with a CAGR of 18.2%. The lithium-ion battery market could grow to $377.6 billion by 2035. You help drive this growth by supporting sustainable practices and resource efficiency.
Ongoing research and development in lithium batteries focus on enhancing sustainability and reducing environmental impact. You play a part in building a cleaner future by choosing and supporting innovative battery technologies.
You see that lithium battery manufacturing involves careful steps and strict quality control.
Battery safety and reliability depend on strong reliability management throughout production and disposal.
Key points to remember:
- Innovations in cell finishing improve battery lif
-

 May.2025.12.22What is a Nickel Cadmium Battery and How Does It WorkLearn More
May.2025.12.22What is a Nickel Cadmium Battery and How Does It WorkLearn More -

 May.2025.12.22How to clean battery corrosion?Learn More
May.2025.12.22How to clean battery corrosion?Learn More -

 May.2025.12.2021700 Battery: Meaning, Comparison with 18650, and How to Choose the Best QualityLearn More
May.2025.12.2021700 Battery: Meaning, Comparison with 18650, and How to Choose the Best QualityLearn More -

 May.2025.12.19Medical Device 18650 Rechargeable Battery: What Buyers Must Evaluate?Learn More
May.2025.12.19Medical Device 18650 Rechargeable Battery: What Buyers Must Evaluate?Learn More -

 May.2025.12.19Common voltage types of lithium polymer batteries for different applicationsLearn More
May.2025.12.19Common voltage types of lithium polymer batteries for different applicationsLearn More